Answer: Option (2) is the correct answer.
Step-by-step explanation:
It is known that tap water acts as an electrolyte because there are a number of ions present in it. As electricity is the flow of ions or electrons therefore, a solution which contains the ions is able to conduct electricity.
For example, ions present in tap water are
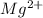 ,
,
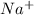 ,
,
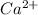 etc.
etc.
Thus, we can conclude that tap water conducts electricity because tap water has many ions dissolved in it, which are responsible for the conduction of electricity.