Answer:
32.04°C will be the final temperature of the solution.
Step-by-step explanation:
Moles of potassium chloride = 0.200 mol
MAs sof KCl= 0.200 mol × 74.5 g/mol= 14.9 g
Enthalpy of solvation of potassium nitrate =
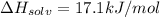
Energy released when 0.200 moles of KCl is dissolved in water = Q
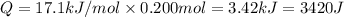
(1 kJ = 1000 J)
Heat released on dissolving 0.200 moles of KCl is equal to heat absorbed by water = Q
Mass of solution , m= 80.0 g +14.9 g = 94.9 g
Specific heat of water = c = 4.184 J/g°C
Initial temperature of the water =
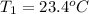
Final temperature of the water =
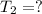
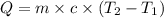
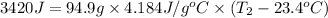
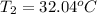
32.04°C will be the final temperature of the solution.