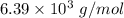Answer:
molar mass is
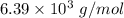
Step-by-step explanation:
Osmotic pressure is related with concentration as follows:

Where, C is concentration or molarity , R is gas constant and T is temperature.
Osmostic pressure given is 0.0766 atm
R is
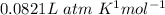
T = 25 + 273 = 298 K
Rearrange the above equation to calculate concetration of the solution as follows:
C = P/RT
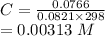
molarity = moles/volume in L
moles = molarity × volume in L
volume = 5.00 mL = 0.005 L
moles = 0.00313 × 0.005
=

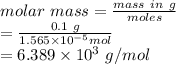
Therefore, molecular mass of protein is