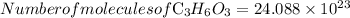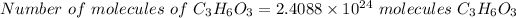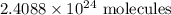 are there in
are there in
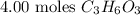
Step-by-step explanation:
One mole =
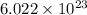 (Applicable to the substances like ions, molecules, or atoms). This specified number is called as Avogadro's constant or number. This idea helps us to convert number of moles of a substance to the number of molecules, multiply moles by Avogadro's number,
(Applicable to the substances like ions, molecules, or atoms). This specified number is called as Avogadro's constant or number. This idea helps us to convert number of moles of a substance to the number of molecules, multiply moles by Avogadro's number,
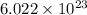
Given:
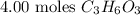
To find the number of molecules in it
By using Avogadro’s number, convert given moles into molecules as below,
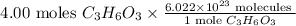
By solving the above, we get