Answer:
1
Step-by-step explanation:
Trigonal planar geometry is shown by the compounds where hybridization of central atom is
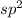 .
.
In
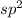 hybridization, three hybrid orbitals are equally spaced at an angle of 120°.
hybridization, three hybrid orbitals are equally spaced at an angle of 120°.
Some of the compounds having
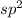 hybridization are
hybridization are
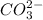 ,
,
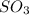 , etc
, etc
In
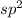 hybridizationm, one s-orbital and 2 p-orbitals are involved.
hybridizationm, one s-orbital and 2 p-orbitals are involved.
Total no. of orbitals present in p-subshell is 3.
As 2 is involved in
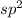 hybridization, therefore no. of unhybridized orbital in
hybridization, therefore no. of unhybridized orbital in
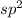 hybridization is 1.
hybridization is 1.