Answer:
The formula of the scandium chloride produced in the reaction is ScCL₃
Step-by-step explanation:
With the given data, you can know the molar relationship between Sc and H₂ (molar ratio) to determine the reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction).
Then:
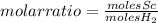
Knowing that:
- mass Sc= 2.12 g
- molar mass of Sc= 44.956 g/mol
- mass H₂= 0.1415 g
- molar mass of H₂= 2 g/mol
and knowing that the number of moles (n) of a compound can be calculated as:
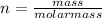
Then:
So:
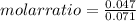
Then it is possible to say that the molar ratio is approximately equal to
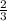 . This indicates that by stoichiometry 2 moles of Sc are needed to produce 3 moles of H₂.
. This indicates that by stoichiometry 2 moles of Sc are needed to produce 3 moles of H₂.
So:
2 Sc + HCl → ScCL₃ + 3 H₂
The law of conservation of matter states that since no atom can be created or destroyed in a chemical reaction, the number of atoms that are present in the reagents has to be equal to the number of atoms present in the products.
Then, balancing the equation so that the same amount of moles of each element on each side of the equation is obtained:
2 Sc + 6 HCl → 2 ScCL₃ + 3 H₂
The formula of the scandium chloride produced in the reaction is ScCL₃