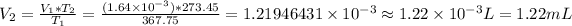Answer:
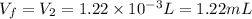
Step-by-step explanation:
Use Charles's law which states: "At constant pressure, the volume occupied by a gas sample is directly proportional to the absolute temperatures they support."
This law can be expressed mathematically as follows:
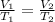
Where:
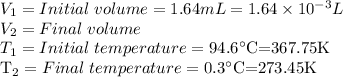
Solving for