Answer: The correct statement is, It behaves as the electron donor.
Step-by-step explanation:
According to the Lewis concept:
A Lewis-acid is defined as a substance that accepts electron pairs.
A Lewis-base is defined as a substance which donates electron pairs.
For example : Acid + Base ⇄ Acid-base adduct
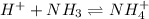
As per question, the characteristic of a Lewis base is that it behaves as the electron donor.
Hence, the correct statement is, It behaves as the electron donor.