Answer:
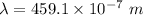 = 459.1 nm
= 459.1 nm
This wavelength corresponds to yellow color and thus gold has warm yellow color.
Step-by-step explanation:
Given that:- Energy = 2.7 eV
Energy in eV can be converted to energy in J as:
1 eV = 1.602 × 10⁻¹⁹ J
So, Energy =
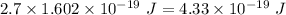
Considering:-
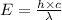
Where,
h is Plank's constant having value
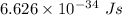
c is the speed of light having value
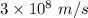
 is the wavelength of the light
is the wavelength of the light
So,
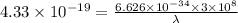
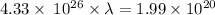
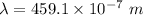 = 459.1 nm
= 459.1 nm
This wavelength corresponds to yellow color and thus gold has warm yellow color.