Answer : The volume of
 gas needed are, 90 L
gas needed are, 90 L
Explanation : Given,
Volume of
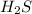 = 60 L
= 60 L
Now we have to determine the volume of
 needed.
needed.
As we know that at STP, 1 mole of gas contains 22.4 L volume of gas.
The given balanced chemical reaction is:
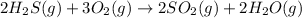
By the stoichiometry we can say that, 2 moles of
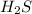 react with 3 moles of oxygen gas to give 2 moles of
react with 3 moles of oxygen gas to give 2 moles of
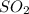 gas and 2 moles of water vapor.
gas and 2 moles of water vapor.
From the balanced reaction we conclude that,
As,
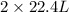 volume of
volume of
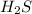 react with
react with
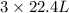 volume of
volume of
 gas
gas
So,
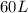 volume of
volume of
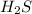 react with
react with
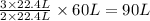 volume of
volume of
 gas
gas
Thus, the volume of
 gas needed are, 90 L
gas needed are, 90 L