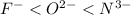Step-by-step explanation:
Lithium, sodium and potassium are all group 1A elements and when we move down a group then there occurs an increase in atomic size of the elements. As lithium is the smallest and potassium being the largest so, when each of them will lose an electron and obtain a positive charge then size of lithium will further decrease.
Therefore, ions are ranked according to their increase in size as follows.
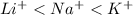
When an atom tends to gain electrons then it acquires a negative charge. This means that size of the atom increases.
So, more is the negative charge present on an atom more will be its atomic size. Therefore, correct order of increasing size for
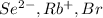 is as follows.
is as follows.
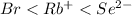
Similarly, order of increasing size of
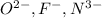 is as follows.
is as follows.