Answer:
(a) Ga (b) Si (d) Te
Step-by-step explanation:
Paramagnetic are those which has unpaired electrons and diamagnetic are those in which all electrons are paired.
(a) Ga
The electronic configuration is -
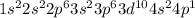
The electrons in 4p orbital = 1 (Unpaired)
Thus, the element is paramagnetic as the electrons are unpaired.
(b) Si
The electronic configuration is -
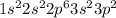
The electrons in 3p orbital = 2 (Unpaired)
Thus, the element is paramagnetic as the electrons are unpaired.
(c) Be
The electronic configuration is -
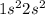
The electrons in 2s orbital = 2 (paired)
Thus, the element is diamagnetic as the electrons are paired.
(d) Te
The electronic configuration is -
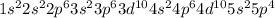
The electrons in 5p orbital = 4 (1 pair and 2 Unpaired)
Thus, the element is paramagnetic as the electrons are unpaired.