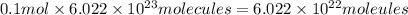Answer:
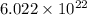 glucose molecules are included in that 100 mL sample.
glucose molecules are included in that 100 mL sample.
Step-by-step explanation:
Concentration of freshly prepared glucose solution = 1 M = 1 mol/L
1 L = 1000 ml
This means that 1 mole of glucose is present in 1000 mL of water.
If we have 100 mL of solution. then number of moles of glucose will be L;
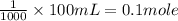
1 mole =
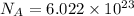 molecules/atoms
molecules/atoms
Number of molecules of glucose in 0.1 mole :
=