Answer:
The vapor pressure of cyclohexane at 81.0°C is 101325 Pa.
Step-by-step explanation:
Given that,
Boiling point = 81.0°C
Atmospheric pressure :
Atmospheric pressure is the force per unit area exerted by the weight of the atmosphere.
The value of atmospheric pressure is
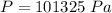
Vapor pressure :
Vapor pressure is equal to the atmospheric pressure.
Hence, The vapor pressure of cyclohexane at 81.0°C is 101325 Pa.