Answer:
38.35 bar
Step-by-step explanation:
We are given that
Temperature=T=36.6 degree Celsius=36.6+273=309.6 K
Given mass of glucose=9.18 g
Molar mass of glucose(
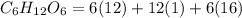 =180 g
=180 g
Mass of c=12 g,mass of hydrogen=1 g, mass of O=16 g
Volume of solution=34.2 mL
Molarity of solution=
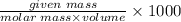
Where volume (in mL)
Molarity of solution=
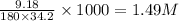
We know that
Osmotic pressure=
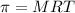
Where M=Molarity of solution
R=Constant=0.08314 Lbar/mol k
T=Temperature in kelvin
Using the formula
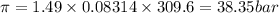
Hence, the osmotic pressure=38.35 bar