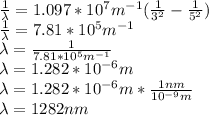Answer:
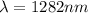
Step-by-step explanation:
The wavelength of the photons emitted due to an atomic electron transition in a hydrogen atom, is given by the Rydberg formula:
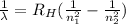
Here
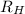 is the Rydberg constant for hydrogen and
is the Rydberg constant for hydrogen and
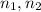 are the lower and higher quantum number for the energy levels of the atomic electron transition, respectively. Replacing the given values and solving for
are the lower and higher quantum number for the energy levels of the atomic electron transition, respectively. Replacing the given values and solving for