Answer:
1.133 moles of chromium (lll) nitrate are produced.
Step-by-step explanation:
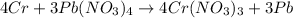
Moles of lead(IV) nitrate = 0.85 mole
According to recation, 3 moles of lead(IV) nitrate gives 4 moles of chromium (III) nitrate.
Then 0.85 moles of lead(IV) nitrate will give:
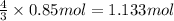 of chromium (lll) nitrate.
of chromium (lll) nitrate.
1.133 moles of chromium (lll) nitrate are produced.