The question is incomplete, complete question is :
For the following chemical reaction, what mass of sodium chromate in grams will be needed to produce 5.17 mol of sodium nitrate?
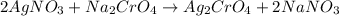
Answer:
418.77 grams of sodium chromate is needed to produce 5.17 mol es of sodium nitrate.
Step-by-step explanation:
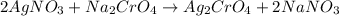
According to reaction, 2 moles of sodium nitarte is obtained from 1 mole of sodium chromate .
Then 5.17 moles of sodium nitarte will be obtained from :
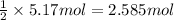 of sodium chromate
of sodium chromate
Mass of sodium chromate :
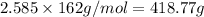
418.77 grams of sodium chromate is needed to produce 5.17 mol es of sodium nitrate.