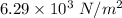Answer:
(a). The maximum loss of water vapor by the person is
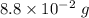
(b). The partial pressure of water vapor is
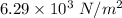
Step-by-step explanation:
Given that,
Temperature = 37.0°C
Volume of air = 2.00 L
Density of vapor = 44.0 g/m³
We need to calculate the maximum loss of water vapor by the person
Using formula of density
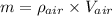
Put the value into the formula
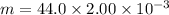
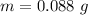
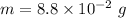
(b). We need to calculate the partial pressure of water vapor
Using formula of pressure

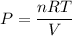
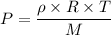
Put the value into the formula
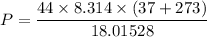
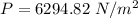
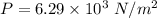
Hence, (a). The maximum loss of water vapor by the person is
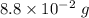
(b). The partial pressure of water vapor is