Answer:
1.126 grams
Step-by-step explanation:
Given that:
Standard Solution = 600 mg O₂/L
The molecular weight of C₆H₁₂O₆ (glucose) = 180.156 g/mol
The mass of O₂ in 1 mole of C₆H₁₂O₆ can be determined as:
C₆H₁₂O₆ = 6 × 16 g ( of one oxygen)
= 96 g
∴ 96 g of O₂ is available in 180.156 gram of C₆H₁₂O₆
Thus C₆H₁₂O₆ required for giving 600 mg = 0.60 g of O₂
∴
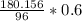
= 1.876625 × 0.6
= 1.125975 g
≅ 1.126 grams
Hence, 1.126 grams of C₆H₁₂O₆ (glucose) will be added to one liter of distilled water in order to get 600mg O₂/L.