Answer:
Step-by-step explanation:
Generally the allowed sub shell (L) in a particular orbit are determined by the principal quantum number (n)
Now for the first orbit , the angular quantum number L (i.e the sub shells) can have a value of only '0'.Considering the orbital n = 2, (L) can have a value '0' and '1' .This mean that the possible transitions from upper levels with
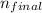 = 2 will be
= 2 will be
n = 6 → n = 2, n = 5 → n= 2, n = 4 → n= 2, n= 3 → n= 2
Generally the second orbit contains, both s(L = 0) and p(L = 1) orbitals. This means that according to the selection rule which states that the change in the quantum number (L) of an allowed transition must be ± 1, Hence the allowed transition in the second orbit are
From n = 3 to n = 2
Generally there are three orbitals that exist in the third orbit, this means that the possible transitions of an electron from n = 3 to n = 2 are
3p → 2s, 3d → 2p , 3s → 2p
Also the possible transition from n = 4 to n = 2 are
4p → 2s , 4d → 2p , 4s → 2p
This then mean that four spectral lines are gotten from these transition as stated below
3p → 2s, 3d → 2p, 3s → 2p, 4s → 2p