Answer:
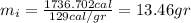
So we need to add 13.46 gr of ice in order to reach the final equilibrium temperature of 49 C
Step-by-step explanation:
For this case we need to use the fact that the sum for all the heats involved in the system are 0, since we assume an equilibrium state.
Data given
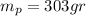 mass of the porcelain cup
mass of the porcelain cup
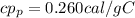 the specific heat for the porcelain cup
the specific heat for the porcelain cup
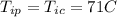 initial temperature for the coffee and the porcelain cup.
initial temperature for the coffee and the porcelain cup.
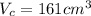 Volume of the coffee.
Volume of the coffee.
We can convert this to m^3 and we got 0.000161m^3 and assuming the density fot the coffee equal to the water 1 Kg/m^3 the mass would be:
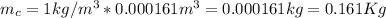
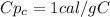 Specific heat for the coffee
Specific heat for the coffee
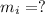 mass of ice required
mass of ice required
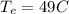 equilibrium temperature
equilibrium temperature
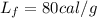 represent the latent heat of fusin since the ice change the state to liquid.
represent the latent heat of fusin since the ice change the state to liquid.
Solution to the problem
Using this formula:
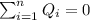
We have this:
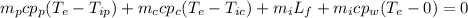
Now we can replace and we have this:
![303 gr *(0.260 cal/g C) (49-71)C + 0.161 gr*(1 cal/g C)(49-71)C +m_i [80 cal/gr+(1cal/g C)(49-0)C]=0](https://img.qammunity.org/2021/formulas/physics/high-school/vz87mcxolmnmgvs67w8x3m8azj2ui42mxn.png)
And now we can solve for
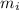 and we have:
and we have:
![-1733.16cal -3.542cal +m_i [129 cal/g]=0](https://img.qammunity.org/2021/formulas/physics/high-school/9nsbjp5rm27ovyarapmmrr9lxo2plaa8tu.png)
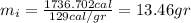
So we need to add 13.46 gr of ice in order to reach the final equilibrium temperature of 49 C