Answer:
Molarity = 0.5 M
Osmolarity = 0.5 x 2 = 1 Osmpl.
Molecules of Cl2 = 6.02 x
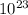 / 4= 1.505 x
/ 4= 1.505 x
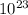 no. of molecules
no. of molecules
Step-by-step explanation:
If we add half mole in 1L volume than molarity will obviously be 0.5 M.
The osmolarity is molarity multiplies by number of dissociates of solute that for CaCl2 are 2. So, 2 x 0.5 = 1
Half will be molecules of Ca and half will be of Cl2 for 0.5M.