Answer:
Current,

Step-by-step explanation:
In this case, we need to find the current in amperes if 1400 Na+ ions flow across a cell membrane in 3.3 μs.
Charge,
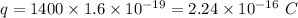
Time taken,
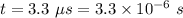
Let I is the current. It is given by total charge per unit time. It is given by :
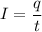
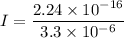

So, the current of
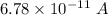 is flowing across a cell membrane. Hence, this is the required solution.
is flowing across a cell membrane. Hence, this is the required solution.