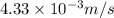Answer : The root mean square speed is,
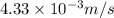
Explanation :
The formula used for root mean square speed is:
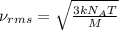
where,
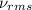 = root mean square speed
= root mean square speed
k = Boltzmann’s constant =
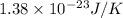
T = temperature = 100 nK =
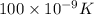
M = atomic mass of cesium = 132.91 g/mole = 0.13291 kg/mole
 = Avogadro’s number =
= Avogadro’s number =
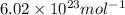
Now put all the given values in the above root mean square speed formula, we get:
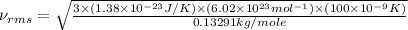
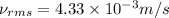
Thus, the root mean square speed is,