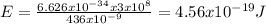Answer:
Step-by-step explanation:
Electromagnetic radiations consist of quanta of energy called photons which have energy, E which is equal to:
E = hν.....................................(1)
where h is the Planck's constant which is
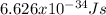 and ν is the frequency of light radiation.
and ν is the frequency of light radiation.
But ν = c/λ ....................................(2)
Putting equation (2) into (1), we have
E = hc/λ..........................................(3)
c is the speed of light (c =
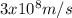 ) while λ is the wavelength of light.
) while λ is the wavelength of light.
Wavelength λ = 436nm =
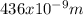
Therefore the energy E of one photon of this light, using equation (3) is