The question is incomplete , complete question is:
Arrange the following H atom electron transitions in order of increasing frequency of the photon absorbed or emitted:
(a) n = 2 to n = 4
(b) n = 2 to n = 1
(c) n = 2 to n = 5
(d) n = 4 to n = 3
Answer:
Hence the order of the transition will be : d < a < c < b
Step-by-step explanation:
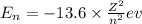
where,
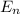 = energy of
= energy of
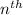 orbit
orbit
n = number of orbit
Z = atomic number
Energy of n = 1 in an hydrogen atom:
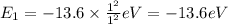
Energy of n = 2 in an hydrogen atom:
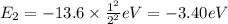
Energy of n = 3 in an hydrogen atom:
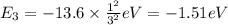
Energy of n = 4 in an hydrogen atom:
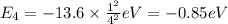
Energy of n = 5 in an hydrogen atom:
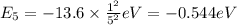
a) n = 2 to n = 4 (absorption)
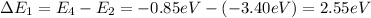
b) n = 2 to n = 1 (emission)
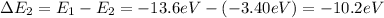
Negative sign indicates that emission will take place.
c) n = 2 to n = 5 (absorption)
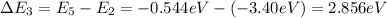
d) n = 4 to n = 3 (emission)
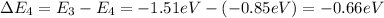
Negative sign indicates that emission will take place.
According to Planck's equation, higher the frequency of the wave higher will be the energy:
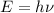
h = Planck's constant
 frequency of the wave
frequency of the wave
So, the increasing order of magnitude of the energy difference :
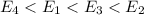
And so will be the increasing order of the frequency of the of the photon absorbed or emitted. Hence the order of the transition will be :
: d < a < c < b