No. of atoms included in 1245 mg is 0.127 *
 atoms.
atoms.
Answer:
Step-by-step explanation:
We know that we can determine the number of atoms in any given amount of sample by dividing the given weight to the atomic mass of the particular sample and then multiplying it with avagadro's number.
So, here the atomic mass of cobalt is 58.9 amu.
Thus,
No.of atoms =
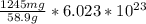
No. of atoms included in 1245 mg is 0.127 *
 atoms.
atoms.
As we know that atomic mass of any element contains avagadro's number of atoms. So in order to find the number of atoms in a given weight of sample, we have to follow the above procedure.
No. of atoms included in 1245 mg is 0.127 *
 atoms.
atoms.