Answer:
The percent yield of a reaction is 48.05%.
Step-by-step explanation:
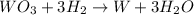
Volume of water obtained from the reaction , V= 5.76 mL
Mass of water = m = Experimental yield of water
Density of water = d = 1.00 g/mL
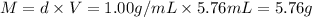
Theoretical yield of water : T
Moles of tungsten(VI) oxide =
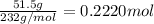
According to recation 1 mole of tungsten(VI) oxide gives 3 moles of water, then 0.2220 moles of tungsten(VI) oxide will give:
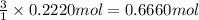
Mass of 0.6660 moles of water:
0.666 mol × 18 g/mol = 11.988 g
Theoretical yield of water : T = 11.988 g
To calculate the percentage yield of reaction , we use the equation:
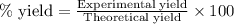
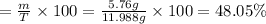
The percent yield of a reaction is 48.05%.