Answer : Yes, theoretical density can be computed from a tiny fundamental unit using the formula
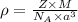 .
.
Explanation :
Nearest neighbor distance, r =
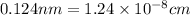
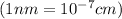
Atomic mass (M) = 55.85 g/mol
Avogadro's number
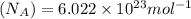
For BCC = Z = 2
Given density =
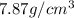
First we have to calculate the cubing of edge length of unit cell for BCC crystal lattice.
For BCC lattice :
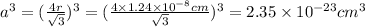
Now we have to calculate the density of unit cell for BCC crystal lattice.
Formula used :
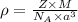 .............(1)
.............(1)
where,
 = density
= density
Z = number of atom in unit cell (for BCC = 2)
M = atomic mass
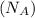 = Avogadro's number
= Avogadro's number
a = edge length of unit cell
Now put all the values in above formula (1), we get
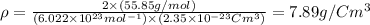
From this information we conclude that, the given density is approximately equal to the given density.
Yes, theoretical density can be computed from a tiny fundamental unit using the formula
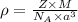 .
.