We need 500 ml of alcohol mixture and 50 ml of water.
Explanation:
The 50% concentration of the final solution is a result of mixing the 55% concentration with water, which is 0% concentration. Multiply the concentration of each liquid times the # of ml you will use, add these and divide by the total ml to get 50%
x = the number of ml of the 55% alcohol mixture
y = the number of ml of water
x + y = 550
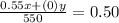
Again, the 0 above represents the water which is 0% concentration
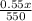 = 0.50
= 0.50
0.55 x = 275
x =
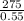 = 500
= 500
plug 500 into the equation x + y = 550
500 + y = 550
y = 50
Use 500 ml of the 55% solution and 50 ml of water.