Answer:
1) 108.27 grams of potassium nitarte must be added to 275 mL of water to produce a solution that freezes at -14.5°C.
2) 1.73 is the actual value of the van't Hoff factor, i.
Step-by-step explanation:
1) Formula used depression in freezing point ;
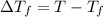
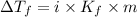
where,
 =Freezing point of solution
=Freezing point of solution
T = Freezing point of water
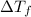 =depression in freezing point =
=depression in freezing point =
i = van't Hoff factor of solute
 = freezing point constant
= freezing point constant
m = molality of solution
We have :
 of water = 1.86°C/m ,
of water = 1.86°C/m ,
Molality of solution = m = ?
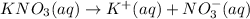
i = 2
Freezing point of solution =
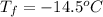
Freezing point of pure water = T = 0°C
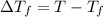
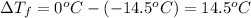
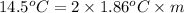
m = 3.898 molal
3.898 moles of potassium nitrate is dissolved in 1 kg of water or 1000 g of water.
Volume of water , V= 275 ml
Mass of water = m
Density of water= d = 1 g/mL
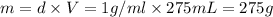
Here, 3.898 moles of potassium nitrate is dissolved in 1 kg of water or 1000 g of water. Then moles of potassium nitarte present in 275 grams of water is :
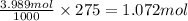
Mass of 1.072 moles of potassium nitrate :
1.072 mol × 101 g/mol = 108.27 g
108.27 grams of potassium nitarte must be added to 275 mL of water to produce a solution that freezes at -14.5°C.
2) Formula used an Elevation in boiling point;
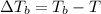
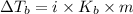
where,
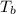 =boiling point of solution
=boiling point of solution
T = boiling point of water
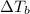 =Elevation in boiling point =
=Elevation in boiling point =
i = van't Hoff factor of solute
 = Boiling point constant
= Boiling point constant
m = molality of solution
of the solution
We have :
 of water = 0.512°C/m ,
of water = 0.512°C/m ,
Molality of solution = m = 3.90 m
i =?
The boiling point of pure water = T = 100.00°C
The boiling point of solution =
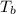 = 103.45°C
= 103.45°C
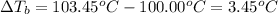
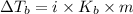
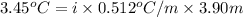
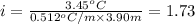
1.73 is the actual value of the van't Hoff factor, i.