Answer:
The fraction of the electrons from the first sphere that must be transferred to the second sphere is 2.2687 X 10¹⁶ Electrons
Step-by-step explanation:
From coulomb's law;
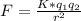
where;
F is the electrostatic force between the two spheres = 200 X 10³ N
k is coulomb's constant = 8.99 X 10⁹ Nm²/C²
q₁ is the charge on first sphere
q₂ is the charge on second sphere
r is the distance between the two spheres = 77cm = 0.77m
Since the two sphere have equal mas, the magnitude of their charge will be same = q
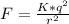
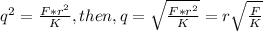
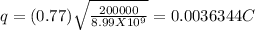
q = 3.6344 X 10⁻³C
The magnitude of charge on each sphere is 3.6344 X 10⁻³C
To find the number of electrons transferred
N = q/e
where;
N is the number of electrons transferred
q is the charge on each sphere
e is the charge on one electron = 1.602 X 10⁻¹⁹ C
N = (3.6344 X 10⁻³)/(1.602 X 10⁻¹⁹)
N = 2.2687 X 10¹⁶ Electrons
The fraction of the electrons from the first sphere that must be transferred to the second sphere is 2.2687 X 10¹⁶ Electrons