"The ability of a base to react with a soluble metal salt" demonstrates characteristic of a base.
Answer: Option D
Step-by-step explanation:
As the sodium hydroxide react with only with some metals. It is a very strong basic compound with a intense solubility in water and hygroscopic in nature. The ability of base is reflected from its potential to react with metals. In the given reaction:
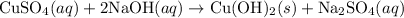
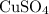 is an acidic property showing salt which is added with sodium hydroxide and this reaction result into a pale blue precipitate of basic copper hydroxide & sodium sulphate which is neutral salt. The reaction is a double displacement and precipitation reaction.
is an acidic property showing salt which is added with sodium hydroxide and this reaction result into a pale blue precipitate of basic copper hydroxide & sodium sulphate which is neutral salt. The reaction is a double displacement and precipitation reaction.