Answer : Yes, all the water will vaporize.
Solution :
We have to determine the total heat absorbed by the sample.
The process involved in this problem are :
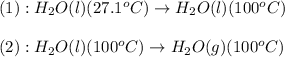
The expression used will be:
![Q=[m* c_(p,l)* (T_(final)-T_(initial))]+m* \Delta H_(vap)](https://img.qammunity.org/2021/formulas/chemistry/college/wythwd1i8wdayn8t6wsmpvk89eb7zinahc.png)
where,
m = mass of sample = 35.0 g
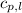 = specific heat of liquid water =
= specific heat of liquid water =
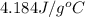
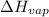 = enthalpy change for fusion =
= enthalpy change for fusion =
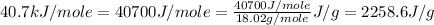
Now put all the given values in the above expression, we get:
![Q=[35.0g* 4.184J/g^oC* (100-27.1)^oC]+35.0g* 2258.6J/g](https://img.qammunity.org/2021/formulas/chemistry/college/qr7lbi9jog9r8chpwihmyrbzrrepl8dogw.png)
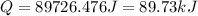
From this we conclude that the calculated heat energy is less than the given heat energy that means all the water will vaporize.
Hence, all the water will vaporize.