Answer:
366.99 kJ
Step-by-step explanation:
Enthalpy of melting of ice = 333.55 kJ
The expression for the calculation of the enthalpy change of a process in which liquid water at 0 °C converts to 40 °C is shown below as:-
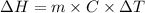
Where,
 is the enthalpy change
is the enthalpy change
m is the mass
C is the specific heat capacity
 is the temperature change
is the temperature change
Thus, given that:-
Mass = 200 g
Specific heat = 4.18 J/g°C
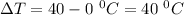
So,
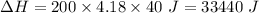
Also, 1 J = 0.001 kJ
So,
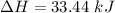
So, total energy absorbed = 333.55 + 33.44 = 366.99 kJ