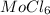Answer:
Step-by-step explanation:
The final product of the metal and chlorine will contain only the total original amount of molybdenum metal (0.1845 g) and chlorine.
Hence, you can determine the amount of chlorine in the compound by subtraction:
- Amount of chlorine in the compound = amount of compound - amount of molybdenum metal
- Amount of chlorine = 0.5936 g - 0.1845 g = 0.4091 g
The empirical formula is the formula that represents the ratio of atoms in the compound using the least positive integer numbers.
Then, you need to convert the masses in grams to number of moles, for which you use the atomic mass of each element.
1. Atomic masses:
- Atomic mass of molybdenum, Mo: 95.94 g/mol
- Atomic mass of chlorine, Cl: 35.453 g/mol
2. Number of moles:
Number of moles = mass in grams / atomic mass
- Number of moles of Mo: 0.1845 g / 95.94 g/mol = 0.001923 mol
- Number of moles of Cl: 0.4091 g / 35.453 g/mol = 0.01154 mol
3. Ratio of moles:
Divide each number of moles by the least number of moles:
- Mo: 0.001923 / 0.001923 = 1
- Cl: 0.01154 / 0.001924 = 6
Ratio:
Hence, the empirical formula is: