Answer: 41.5 min
Explanation:
This problem can be solved with the Radioactive Half Life Formula:
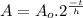 (1)
(1)
Where:
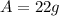 is the final amount of the radioactive element
is the final amount of the radioactive element
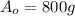 is the initial amount of the radioactive element
is the initial amount of the radioactive element
 is the time elapsed
is the time elapsed
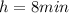 is the half life of the radioactive element
is the half life of the radioactive element
So, we need to substitute the given values and find
 from (1):
from (1):
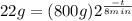 (2)
(2)
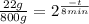 (3)
(3)
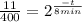 (4)
(4)
Applying natural logarithm in both sides:
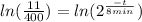 (5)
(5)
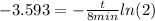 (6)
(6)
Clearing
 :
:
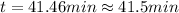 This is the time elapsed
This is the time elapsed