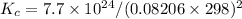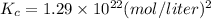Answer:
- Part A: Kc = 0.00573 mol/liter
- Part B: Kc = 1.29 × 10²² (mol/liter)²
Step-by-step explanation:
Part A
1. Chemical equation
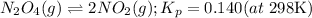
2. Kp in terms of Kc
- Kp it the quilibrium constant in terms of the partial pressures at equilibrium of the gases that react and it is equal to:
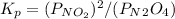
- Kc is the equilibrium constant in terms of the molar concentrations at equilibrum of the compounds that react and it is equal to:
![K_c=[{NO_2]^2/[N_2O_4]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/wllzfvdzbw5hy6hq0llyu63ophkps2qxlf.png)
- Kp and Kc are related by:
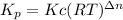
Where,
- R is the universal gas constant, R = 0.08206 atm-liter / K-mol,
- T is the absolute temperature (Kelvin), T = 298 Kand
-
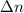 is the difference of coefficients of the chemical equilibrium equation for gases (the number of moles of gases on the right side less the number of moles of gases on the left sid) = 2 - 1
is the difference of coefficients of the chemical equilibrium equation for gases (the number of moles of gases on the right side less the number of moles of gases on the left sid) = 2 - 1
Then, given that you know Kp, you can solve for Kc:
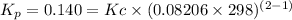
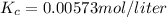
Part B
1. Chemical equation:
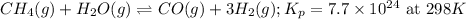
2. Kp in terms of Kc
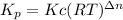
3. Solve for Kc
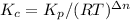
4. Δn
Δn = (1 + 3) - (1 + 1) = 4 - 2 = 2
5. Compute