Answer:
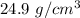
Step-by-step explanation:
Density is found dividing mass by volume. In this case, we treat krypton as a sphere having a volume of
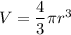 . Given:
. Given:
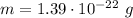
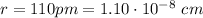
We obtain density of:
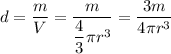
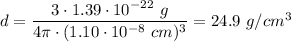
This is not a feasible value for a gas like krypton, its radius is actually not 110 pm.