Answer:
electrons are transferred from the wool shirt to the plastic rod is 4.375 ×
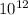 electrons
electrons
Step-by-step explanation:
given data
Amount of charge transferred Q = -0.7 μC = -0.7 ×
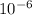 C
C
electrons of charge e = -1.6 ×
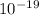 C
C
solution
we apply formula here for total charge of electrons that is
Q = n × e ...................2
here n is no of electron that is
n =

put here value
n =

n = 4.375 ×
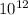 electrons
electrons
so here electrons are transferred from the wool shirt to the plastic rod is 4.375 ×
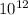 electrons
electrons