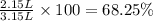Answer:
The percentage of Lake Parsons which has evaporated since it became isolated is 68.24%.
Step-by-step explanation:
The concentration of salt dissolved in Lake Parson = 21.0 g/L
= In 1 L of lake Parson water = 21.0 g of salt
The concentration of salt in several nearby non-isolated lakes = 6.67 g/L
The salt concentration in Lake Parsons before it became isolated = 6.67 g/L
In 1 L of lake water = 6.67 g of salt
If 1 L of lake Parson water has 6.67 g of salt. Then 21.0 grams of salt will be in ;
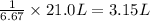
Water evaporated = 3.15 L - 1 L = 2.15 L
The percentage of Lake Parsons which has evaporated since it became isolated: