Answer: 2Liters
Step-by-step explanation:
The expression used will be :
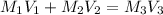
where,
 = concentration of first antifreeze= 60%
= concentration of first antifreeze= 60%
 = concentration of second antifreeze= 10%
= concentration of second antifreeze= 10%
 = volume of first antifreeze = x L
= volume of first antifreeze = x L
 = volume of second antifreeze = 8 L
= volume of second antifreeze = 8 L
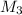 = concentration of final antifreeze solution= 20%
= concentration of final antifreeze solution= 20%
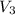 = volume of final antifreeze = (x+8) L
= volume of final antifreeze = (x+8) L
Now put all the given values in the above law, we get the volume of antifreeze added
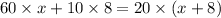
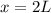
Therefore, the volume of 60% antifreeze solution that must be added is 2L