Answer:
The correct answer is option A.
Step-by-step explanation:
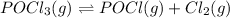
Reducing the partial pressure of chlorine gas means that removal of chlorine gas from the equilibrium will shift the equilibrium in the direction according to Le Chatelier's principle.
On a removal of product from the equilibrium shifts the reaction in the forward reaction for reestablishment.So, when the partial pressure of chlorine is reduced by 50% the equilibrium will shift in forward direction in which
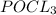 will be consumed to form more chlorine gas.
will be consumed to form more chlorine gas.
Hence, A is correct .