ANSWER:
What is the measured component of the orbital magnetic dipole moment of an electron with the values
(a) ml=3
(b ) ml= −4
a) -278 x
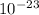 J/T
J/T
b) 3.71 x
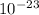 J/T
J/T
Explanation:
a) ml= 3
Цorb,z = ml Цв = - (3) * (9.27e - 24) = -278 x
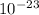 J/T
J/T
b) ml= 3
Цorb,z = ml Цв = - (-4) * (9.27e - 24) = 3.71 x
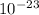 J/T
J/T