Step-by-step explanation:
![ClO^-(aq)+H_2O(l) \rightarrow HClO (aq)+OH^-(aq)\ [fast]](https://img.qammunity.org/2021/formulas/chemistry/high-school/ekfk8pqb7oy9d9v5y2mpsmdtwd4hm9mwb9.png)
![I^-(aq)+HClO(aq) \rightarrow HIO(aq)+Cl^-(aq)\ [slow]](https://img.qammunity.org/2021/formulas/chemistry/high-school/y0mb2026cw3et8c5a1e261yxz3weiynn62.png)
![OH^-(aq)+HIO(aq) \rightarrow H_2O(l)+IO^-(aq)\ [fast]](https://img.qammunity.org/2021/formulas/chemistry/high-school/x4iau5gm7yy7tmq3ju3szh3ixpdjoqfxzi.png)
(a)
Cancelling common terms, the overall reaction is as follows:
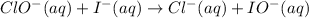
(b)
Chemical species which are generated and consumed during the reaction are called intermediates. Therefore, in the given reaction, HClO(aq), OH-(aq) and HIO(aq) are intermediate species.
(c)
two reactant molecules are involved in each steps, therefore, each steps are bimolecular. Since water is in excess, therefore will not be rate law
Rate law for first step:
![rate=k_1[ClO^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/3ida4sq2e5u1w8hxcjaphwmtlbeeqhh21z.png)
Rate law for second step:
![rate=k_2 [I^-][HClO]](https://img.qammunity.org/2021/formulas/chemistry/high-school/5o1cgzgjez4aaqtmztmcf8n81fcbous2i4.png)
Rate law for third step:
![rate=k_3[OH^-][HIO]](https://img.qammunity.org/2021/formulas/chemistry/high-school/jsgvg8frpsy5lsuhkyygpomfidpom8795n.png)
(d)
Step 2 is slow step, therefore this step will be rate determining step.
So, rate = k [I-] [HClO]
So, it consisted with the actual rate law