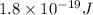Answer:
Step-by-step explanation:
Using Rydberg's Equation for hydrogen atom:
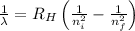
Where,
 = Wavelength of radiation
= Wavelength of radiation
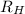 = Rydberg's Constant
= Rydberg's Constant
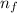 = Higher energy level
= Higher energy level
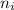 = Lower energy level
= Lower energy level
We have:
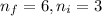
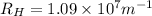
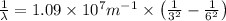
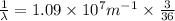
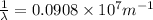
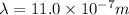
The relation between energy and wavelength of light is given by Planck's equation, which is:
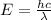
where,
E = energy of the light
h = Planck's constant
c = speed of light
 = wavelength of light
= wavelength of light
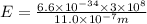
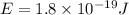
Thus energy of a photon that is ejected from a hydrogen atom when an electron relaxes from the 6th to the 3rd energy level is