Answer: The equilibrium concentration of hydrogen gas is 0.0269 M
Step-by-step explanation:
The chemical reaction follows the equation:
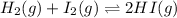
At t = 0 0.044M 0.044M 0.177M
At
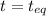 (0.044-x)M (0.044-x)M (0.177+x)M
(0.044-x)M (0.044-x)M (0.177+x)M
The expression for
 for the given reaction follows:
for the given reaction follows:
![K_c=([HI]^2)/([H_2]* [I_2])](https://img.qammunity.org/2021/formulas/chemistry/college/3bneqzi54v5uf5j9r721a6z4azqh1ccv40.png)
We are given:
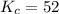
Putting values in above equation, we get:
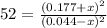
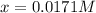
Hence, the equilibrium concentration of hydrogen gas is (0.044-x) M =(0.044-0.0171) M= 0.0269 M