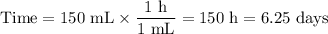Answer:
1. 389 kJ; 2. 7.5 µg; 3. 6.25 days
Step-by-step explanation:
1. Energy required
The water is converted directly from a solid to a gas (sublimation).
They don't give us the enthalpy of sublimation, but
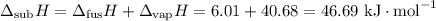
The equation for the process is then
Mᵣ: 18.02
46.69 kJ + H₂O(s) ⟶ H₂O(g)
m/g: 150
(a) Moles of water
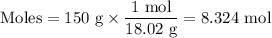
(b) Heat removed
46.69 kJ will remove 1 mol of ice.
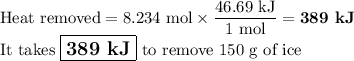
2. Mass of water vapour in the freezer
For this calculation, we can use the Ideal Gas Law — pV = nRT
(a) Moles of water
Data:
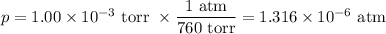
V = 5 L
T = (-80 + 273.15) K = 193.15 K
Calculation:

(b) Mass of water
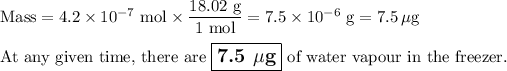
3. Time for removal
You must remove 150 mL of water.
It takes 1 h to remove 1 mL of water.