Answer:
2.49 × 10⁶ molecules
Step-by-step explanation:
Given data
- Pressure (P): 9.25 × 10⁻¹⁴ atm
- Volume (V):
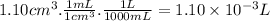
We can calculate the moles of gas using the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 9.25 × 10⁻¹⁴ atm × 1.10 × 10⁻³ L / (0.0821 atm.L/mol.K) × 300.0 K
n = 4.13 × 10⁻¹⁸ mol
1 mole contains 6.02 × 10²³ molecules (Avogadro's number). The number of molecules in 4.13 × 10⁻¹⁸ moles is:
4.13 × 10⁻¹⁸ mol × (6.02 × 10²³ molecule/1 mol) = 2.49 × 10⁶ molecule